CReDO Science, LLC, a company dedicated to the development of solutions for unmet medical needs through cannabis-based therapies, strives to commercialize patented products generated from our investigation of the cannabis plant and the endocannabinoid system (ECS). CReDO Science is offering its expertise to the pharmaceutical industry for the development of optimized cannabis-based medicines not limited to but focused on the following priority indications:
- Neurodegenerative disease (specifically ALS and Alzheimer disease)
- Brain trauma (post-concussive syndromes and chronic traumatic encephalopathy)
- Endometriosis
- Hyperemesis gravidarum
- Anxiety disorders (including PTSD)
- Intractable depression
- Smoking cessation
- Opioid and other addictions
- Orphan indications
While two cannabis-based medicines have gained regulatory approval to date, it is our feeling that no currently available formulations have been truly optimized. We will leverage our knowledge of the unique pharmacology of cannabis components to formulate therapeutic options combining cannabinoids and terpenoids to maximize synergistic potential of the entourage effect in a manner that will fulfill regulatory requirements of efficacy, safety and consistency.
Why Engage CReDO Science?
The typical pharmaceutical development course for new chemical entities (NCE) often takes 10-12 years and requires capital expenditures of $800 million to $1.2 billion. Even for neurological orphan indications that might gain regulatory approval with a single well-designed Phase II clinical trial, this process still often takes 8 years and requires a capital investment of at least $28 million.
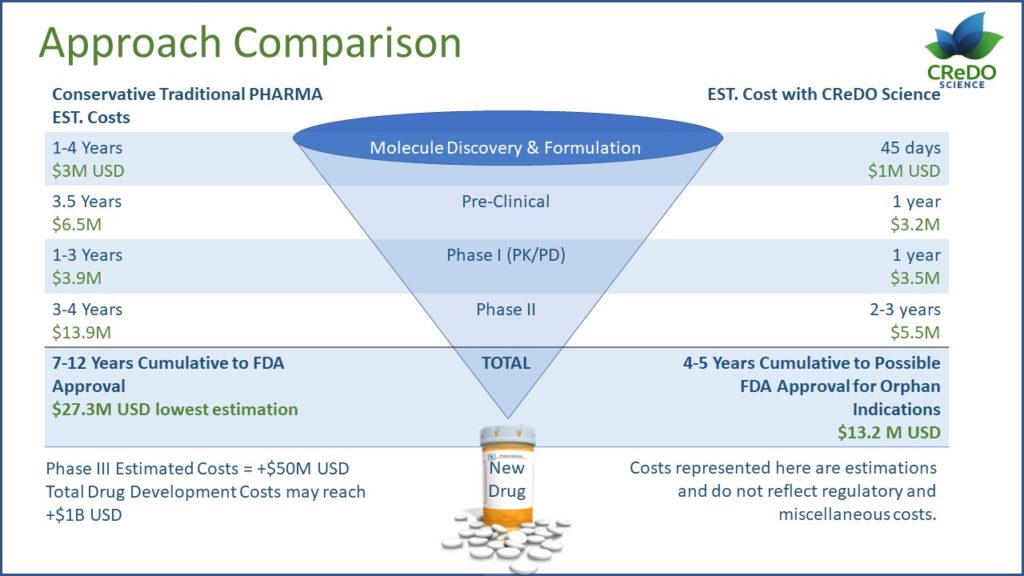
In contrast to NCE development and its risks of unexpected toxicity, cannabis-based medicines portend to provide a development pathway of 4-5 years, using materials whose adverse event profiles are well defined and understood and with a capital outlay as low as $17 million for orphan indications, e.g. amyotrophic lateral sclerosis (ALS).
The CReDO Science Process
In exchange for an upfront technical fee for formulation disclosure with royalty to follow upon sale after successful Phase II randomized controlled trial (RCT) or regulatory approval, CReDO Science will be available to its pharmaceutical partners throughout the FDA- approval process for additional consultation at negotiated rates for input on study design, including inclusions, exclusions, primary and secondary outcome measures, pharmacovigilance and regulatory issues.
CReDO Science has an established international network of world-class experienced clinical and basic science researchers available for additional collaboration and consultation, as well as a dedicated contract research organization (CRO) with extensive clinical trial experience with cannabis.
Expertise
Dr. Russo, CEO and founder of CReDO Science, is a board-certified pediatric and adult neurologist, who spent 20 years in clinical practice and has worked in the cannabis industry for over 25 years, including an 11 years as Senior Medical Advisor to GW Pharmaceuticals as study physician and medical monitor for over 20 Phase I-III clinical trials of Sativex® and Epidiolex®. He has been called the top medical cannabis formulator in the world. His mantra to his clients and potential clients is, “Optimal formulation is foundational to drug development success.”
Time Savings
Choosing CReDO Science for formulation/strategic guidance services reduces the drug discovery phase from 1-3 years to a 30-60-day process and will expedite the pre-clinical and clinical processes.
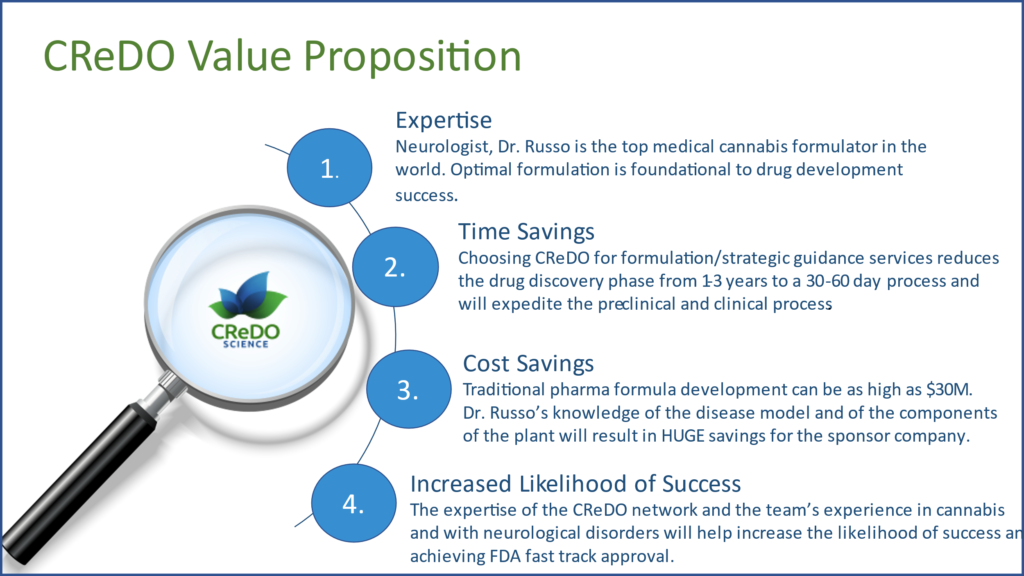
Cost Savings
Traditional pharmaceutical formula development can easily exceed $30M. Dr. Russo’s knowledge of the disease model and of the components of the cannabis plant will result in substantial savings for the sponsor company.
Increased Likelihood of Success
The expertise of the CReDO Science network and the team’s experience in cannabis and with neurological disorders will help increase the likelihood of success and achieving FDA and other regulatory fast-track approval.
Partner Requirements
CReDO Science seeks highly qualified companies with whom to cooperate that have the scientific acumen, regulatory process experience and the financial strength to carry clinical investigations through successful Phase II clinical trials and IND status.
Consulting agreements are available for review on request.
Application Process
Please send inquiries and proposals to:
Nishi Whiteley, COO
nw at credo-science dot com
